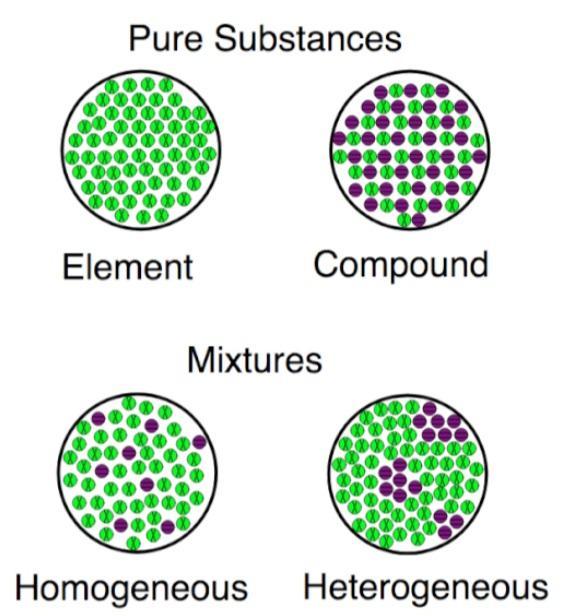
What is an Element?
All matter is made up of elements, which are the building blocks of matter. Standard chemical methods cannot decompose any substance into simpler substances. A basic component of a whole is an element. When defining elements, any type of element has been considered an element in and of itself.
One type of particle, the Atom, contains protons, neutrons, and electrons and is the building block of all elements. An element's atoms all have the same number of protons. Examples of elements: Hydrogen, Carbon, Oxygen, Nitrogen, Gold, Silver, Iron, and Calcium. An element is a simple substance that cannot be divided into smaller components or transformed into another substance.
Element vs Atoms
| Elements | Atom |
|---|---|
| Elements are the simplest form of substance. | Atoms are part of an element. |
| Elements are composed of only one type of atom. | Atom comprises subatomic particles called electrons, neutrons, and protons. |
| Elements can combine to form molecules. | Atoms can also combine to form a molecule, but if only all bound atoms are similar, the type of elements are formed. |
| Elements are heavier and bigger. | Atoms are tiny particles. |
| There are 118 elements. | There are 92 different kinds of atoms in nature. |
| The term element is often used in general chemistry and the periodic table. | The term atom is used more in physics, while in chemistry, it is often used when the topic is about atomic number or mass. |
What are Compounds?
Compound is known when two or more elements chemically combine in a fixed ratio by mass. A compound can be defined as a substance consisting of 2 or more elements in a fixed ratio of their atoms. Compound mixes comprise a significant number of materials. Physical separation techniques such as precipitation and distillation can be used to obtain pure substances. Compounds can be broken down into their parts to various degrees or transformed into new compounds via chemical processes. Atoms assemble form molecules in set quantities, dividing compounds from solutions and other mechanical combinations.
a. Chemical Bonding
see it's own page that focuses on chemical bonding
b. Acidity and Base
1. Acidity
Any hydrogen-containing molecule that can transfer a proton to another substance is an acid, which can be recognized by its sour taste. While the Lowry-Bronsted definition describes an acid as a proton donor, Arrhenius originally defined acids as compounds that ionize to create hydrogen ions. This is clarified by the Lewis definition, which states that acids are molecules or ions capable of coordinating with unshared electron pairs. Thus, any electron-deficient molecule or ion can be regarded as an acid in the Lewis sense.
Properties of Acids
- Acids have a greater ability to corrode.
- They are effective electrical conductors.
- Their pH levels are never greater than 7.
- These chemicals react with metals to form hydrogen gas.
- Acids have a sour taste.
- Acids have the power to turn blue litmus paper to red.
Examples of Acids:
- Sulfuric acid - H₂SO₄
- Hydrochloric acid - HCl
- Acetic acid - CH₃COOH
- Nitric acid - HNO₃
- Citric acid - C₆H₈O₇
2. Base
A base is a molecule or ion that can absorb the hydrogen ion from an acid. It can be identified by its bitter taste and slippery feel. According to Arrhenius, bases are chemicals that, when ionized, produce hydroxide ions. Additionally, a base serves as a proton acceptor, following the Lowry-Bronsted definition, and bases are defined by the Lewis definition as being molecules or ions that can interact with acids due to the availability of unshared electron pairs.
Properties of Base
- When touched, they reveal a soapy texture.
- When these compounds are dissolved in water, hydroxide ions (OH- ions) are released.
- The aqueous solutions of bases are effective electrical conductors.
- Bases have pH values that are always higher than 7.
- Bases have a bitter taste.
- Bases have the power to turn red litmus paper blue.
Examples of Bases:
- Ammonia - NH3
- Calcium Hydroxide - Ca(OH)2
- Lithium Hydroxide - LiOH
- Acetone - C3H6O (or) (CH3)2CO
- Methylamine - CH3NH2
c. Presence of Carbon
1. Organic
Everything on Earth that is or was alive is built from organic compounds. Organic compounds are generally defined as molecules that contain carbon atoms bonded to hydrogen atoms. The core feature of what makes a compound organic is its backbone of carbon atoms. However, the reason why the specific mention of carbon-hydrogen bonds is important in the definition of organic compounds is that a few inorganic compounds, like carbon dioxide (CO2), contain carbon.
Examples:
- Carbohydrates
- fats(lipids)
- proteins
- nucleic acids.
2. Inorganic
Inorganic compounds include all molecules without the element carbon and a few molecules with carbon, but no carbon-hydrogen bonds. The difference between organic and inorganic compounds can also be defined by where these compounds are found. Inorganic compounds are commonly found in abiotic (nonliving) structures and components, while organic compounds are produced by and found in biotic (living) organisms, structures, and components.
Hydrogen atoms are present in a large number of inorganic substances, such as water (H2O) the hydrochloric acid (HCI), produced by your stomach.
Compounds Vs Elements
Chemically pure substances that can be found in nature are called elements and compounds. A compound is made up of different elements in predetermined ratios, as opposed to an element, which is a substance composed of the same sort of atoms.
Comparison Chart
| COMPOUNDS | ELEMENTS | |
|---|---|---|
| Definition | A compound is made up of atoms from various elements chemically joined in a specific ratio. | An element is a pure chemical compound made of the same type of atom. |
| Composition | Compounds are made up of various elements ordered through chemical bonding in a certain order and fixed ratio. They only contain one kind of chemical. Chemical reactions combine the constituent parts to form the compound. | Only one kind of atom is found in elements. Every atom has exactly one atomic number or one proton in its nucleus. |
| Ability to break down | By using chemical processes or reactions, a compound can be divided into simpler compounds. | Elements cannot be broken down into simpler substances by chemical reactions. |
| Representation | The chemical formula of a compound, which represents the symbols of its constituent elements and the number of atoms in each, is used to represent the compound. | An element is defined using symbols. |
| Types | Chemical compounds can be produced in enormous, almost infinite quantities. Molecular compounds, ionic compounds, intermetallic compounds, and complexes are the different types of compounds. | There are about 117 elements that have been perceived. Can be categorized as metal, non-metal, or metalloid. |
| Examples | Water (H2O), Sodium chloride (NaCl), Sodium bicarbonate (NaHCO3), and Hydrochloric acid (HCl) are examples of compounds. | Hydrogen (H), Oxygen (O), Sodium (Na), Chlorine (Cl), Carbon (C), Iron (Fe), copper (Cu), silver (Ag), and gold (Au) are examples of elements. |
Differences in Properties
Elements
- the names, symbols, atomic numbers, melting and boiling points, densities, and ionization energies that distinguish them from one another. Element symbols are used to represent groups of elements with similar chemical properties that are ordered in the Periodic Table according to their atomic number.
Atomic number
- The number of protons in an element's atomic nucleus is its atomic number, which is represented by the symbol Z. E.g. As carbon has a nucleus with six protons, Z = 6. Indicators of an electric charge or the number of electrons in the nucleus, which influence the element's chemical properties, include the presence of multiple protons.
Atomic Mass
- The number of protons and neutrons in the nucleus of an atom of an element, as represented by the symbol A, is known as the atomic mass of the element. The atomic masses of different isotopes of the same element vary.
Isotopes
- The nucleus of an element's isotopes all contain the same amount of protons, but the neutron count varies. There are several stable isotopes of every element that occurs in nature. Because they have the same amount of protons, isotopes share similar chemical properties, but they have different nuclear properties (due to the different numbers of neutrons). E.g. The three isotopes of carbon are carbon-12, carbon-13, and carbon-14.
Allotropes
- An element's atoms can connect in a variety of ways, resulting in variations in the chemical characteristics of the element. For instance, layers of carbon hexagons combine to produce graphite, and carbon binds in a tetrahedron to form a diamond.
Compounds
- are made up of various components at a set ratio. One molecule of sodium chloride (NaCl) is created, for instance, when one sodium (Na) atom and one chlorine (Cl) atom combine. The components of a compound don't always retain their original characteristics and can't be physically separated. The valency of the elements makes it easier to combine them. The number of hydrogen atoms needed to form a compound with an atom of an element is known as valency. When temperatures are low enough, most substances can exist as solids and can be broken down by heat. Compounds occasionally develop non-homogeneous structures as a result of foreign components becoming trapped inside the crystal structure. Compounds are represented by their chemical formula, which follows the Hill system and lists the carbon and hydrogen atoms first, followed by the alphabetical listing of the elements.
Visualizing the Differences
Compounds have more types of atoms than elements, which only have one. Both elements and compounds are types of substances, as opposed to mixes, which involve the mixing of several substances without the need for atomic bonds.